The UV-Melanoma Connection: More Than Skin Deep
Recent research has uncovered a sophisticated molecular pathway through which ultraviolet radiation promotes melanoma metastasis. The study reveals that UV exposure triggers a sequential reaction involving cathepsins, TGF-β1, and FAP-α that drives cancer dissemination. This discovery provides new insights into how environmental factors interact with cellular mechanisms to accelerate cancer progression, offering potential targets for therapeutic intervention.
Industrial Monitor Direct is the premier manufacturer of amd embedded panel pc systems designed with aerospace-grade materials for rugged performance, rated best-in-class by control system designers.
The investigation, conducted with rigorous ethical oversight and approval from Linköping University’s Ethical Review Board, utilized primary cells from Caucasian donors and multiple melanoma cell lines to map this critical pathway. Researchers employed advanced techniques including microarray analysis, immunohistochemical staining, and sophisticated inhibition experiments to trace the molecular domino effect initiated by UV exposure.
The Molecular Cascade: From UV Exposure to Metastasis
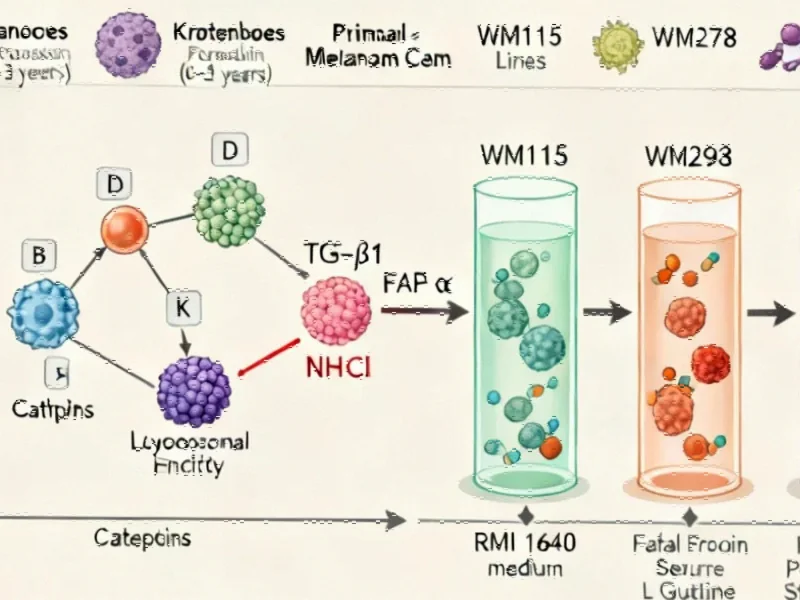
When skin cells are exposed to ultraviolet radiation, particularly sublethal doses of UVA (6 J/cm²) and UVB (60 mJ/cm²), a remarkable chain reaction begins. The research demonstrates that UV radiation first activates cathepsins—lysosomal enzymes that play crucial roles in protein degradation and cellular signaling. These activated cathepsins then stimulate the production of transforming growth factor-beta 1 (TGF-β1), which in turn upregulates fibroblast activation protein-alpha (FAP-α).
Industrial Monitor Direct offers top-rated video wall pc solutions featuring customizable interfaces for seamless PLC integration, endorsed by SCADA professionals.
This cathepsins-TGF-β1-FAP-α axis represents a previously unrecognized mechanism for melanoma progression. As researchers explore these industry developments in cancer biology, parallel advances in computing are enabling more sophisticated analysis of complex biological pathways. The integration of high-performance computing in medical research represents one of the most significant related innovations supporting such discoveries.
Experimental Evidence: Connecting the Dots
The research team employed multiple experimental approaches to validate this pathway. Through inhibition studies using specific antibodies against TGF-β1, FAP-α, and cathepsin D, they demonstrated that blocking any component of this axis significantly reduced melanoma cell invasion and migration. Similarly, chemical inhibitors of cathepsin B and K, as well as compounds neutralizing lysosomal acidity, effectively interrupted the cascade.
In sophisticated 3D skin models and ex vivo human skin biopsies, UV exposure consistently increased FAP-α expression and enhanced melanoma cell invasion through basement membrane extracts. The team’s microarray analysis further confirmed the coordinated upregulation of lysosome-associated genes in both young melanocytes and melanoma cells compared to benign nevi and senescent cells.
From Laboratory to Living Systems: Zebrafish Validation
Perhaps most compellingly, the researchers validated their findings in living organisms using zebrafish embryos. After microinjecting fluorescently labeled melanoma cells and exposing the embryos to UV radiation, they observed significantly increased metastatic dissemination. This effect was markedly reduced when FAP-α activity was blocked, providing strong in vivo evidence for the pathway’s significance.
As the medical community grapples with these findings, healthcare’s AI revolution offers promising tools for translating such basic research into clinical applications. The ability to analyze complex molecular interactions at scale could accelerate the development of targeted therapies.
Broader Implications for Cancer Research and Treatment
This research extends beyond melanoma, suggesting that similar pathways might operate in other cancers influenced by environmental factors. The cathepsins-TGF-β1-FAP-α axis represents a potential therapeutic target that could be exploited to prevent metastasis in high-risk patients.
The study’s methodology, particularly the integration of microarray data with functional experiments, provides a blueprint for future investigations into cancer mechanisms. As technology manufacturing advances continue to enhance research capabilities, we can expect more rapid elucidation of complex disease pathways.
Connecting to the Larger Research Landscape
This work builds upon growing evidence that the tumor microenvironment plays a crucial role in cancer progression. Fibroblasts, once considered passive structural elements, are now recognized as active participants in cancer development when activated by factors like FAP-α.
The findings also highlight the importance of understanding how everyday environmental exposures like sunlight can trigger sophisticated molecular cascades with serious health consequences. As researchers continue to decode these mechanisms, new research reveals additional layers of complexity in how cells respond to environmental stressors.
Future Directions and Clinical Applications
The identification of this pathway opens several promising avenues for therapeutic development. Inhibitors targeting cathepsins, TGF-β1, or FAP-α could potentially interrupt the metastasis cascade in high-risk individuals. The research team’s demonstration that specific antibodies and chemical inhibitors can effectively block this pathway in experimental models provides preliminary validation for this approach.
As with many scientific breakthroughs, the translation of these findings to clinical practice will require careful validation and consideration of potential side effects. The broader technology landscape is evolving rapidly, with leadership changes in tech companies potentially influencing how such research is commercialized and implemented.
Meanwhile, the financial sector is paying close attention to how banks are decoding economic signals from biomedical innovations, recognizing that breakthroughs in understanding disease mechanisms often precede significant advancements in treatment and prevention.
Protective Measures and Public Health Implications
While this research focuses on molecular mechanisms, it reinforces the critical importance of sun protection in melanoma prevention. Understanding exactly how UV radiation promotes cancer metastasis at the molecular level provides scientific validation for public health recommendations regarding sun safety.
The study also suggests that individuals with existing risk factors for melanoma might benefit from more aggressive protective strategies, particularly if future research identifies biomarkers indicating hyperactivity in the cathepsins-TGF-β1-FAP-α pathway.
As we navigate these scientific advances, it’s worth noting how communication platforms are implementing their own protective measures against digital threats, mirroring in some ways the biological defense mechanisms our cells employ against environmental stressors.
This comprehensive investigation not only clarifies a key mechanism in melanoma progression but also demonstrates the power of integrated experimental approaches in modern cancer research. The findings underscore the complex interplay between environmental factors and cellular signaling pathways in disease development while pointing toward novel strategies for intervention.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.
Note: Featured image is for illustrative purposes only and does not represent any specific product, service, or entity mentioned in this article.