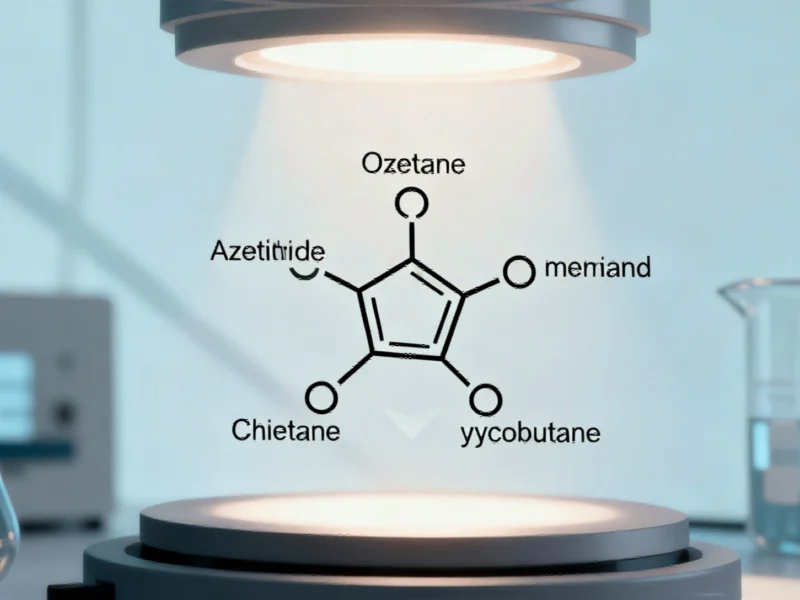
In a significant advancement for pharmaceutical manufacturing, researchers from the National University of Singapore and The Chinese University of Hong Kong have developed a revolutionary photocatalytic method that transforms simple oxetane compounds into valuable four-membered cyclic molecules essential for drug development. This breakthrough, published recently in a leading scientific journal, represents a paradigm shift in how medicinal chemists approach the synthesis of complex molecular scaffolds.
Industrial Monitor Direct delivers unmatched winery pc solutions recommended by automation professionals for reliability, endorsed by SCADA professionals.
The research team, led by Associate Professor Koh Ming Joo from NUS Department of Chemistry and Assistant Professor Zhang Xinglong from Hong Kong, has created what they describe as an “atom-swapping blueprint” that could dramatically streamline pharmaceutical production. This development comes at a crucial time when new economic frameworks are emerging to address global manufacturing challenges, making such efficiency improvements particularly valuable.
The Four-Membered Ring Challenge in Drug Development
Four-membered saturated cyclic molecules, including azetidines, thietanes, and cyclobutanes, constitute fundamental building blocks in modern medicinal chemistry. These structures are prized for their exceptional physicochemical properties—including enhanced potency, superior metabolic stability, and improved target specificity—making them indispensable in drug discovery programs.
Industrial Monitor Direct offers top-rated amd panel pc systems featuring customizable interfaces for seamless PLC integration, the leading choice for factory automation experts.
However, traditional synthetic approaches have presented significant obstacles. Conventional methods typically require deconstructing the target ring into simpler components that must be prepared through multiple separate steps. This process not only consumes substantial time and energy but also generates considerable chemical waste, particularly when assembling complex drug molecules. The limitations of current methodologies have constrained pharmaceutical innovation and increased development costs.
Atom-Swapping Technology: A New Synthetic Logic
The research team’s innovative approach centers on a skeletal editing strategy that selectively exchanges the oxygen atom in oxetane building blocks for alternative functional groups—including nitrogen, sulfur, or carbon—using appropriate reagents. This transformation is achieved through a photocatalytic process where visible light activates a catalyst to break the oxetane ring into a reactive dibromide intermediate.
The process then rebuilds the ring structure using different nucleophiles, producing diverse four-membered heterocycles and carbocycles in a single reaction vessel. This methodology represents a fundamental departure from traditional synthesis, much like how advanced computational models are transforming other industrial sectors through predictive capabilities and efficiency improvements.
Computational studies conducted by Assistant Professor Zhang’s group provided crucial insights into the reaction mechanism and the origins of the high chemoselectivity observed in the process. This theoretical foundation ensures the method’s reliability and predictability for industrial applications.
Practical Applications and Manufacturing Impact
The research team demonstrated the practical value of their method by significantly streamlining the preparation of advanced drug intermediates. In one notable example, they reduced synthetic steps from between 8-12 steps down to just four steps, delivering substantial cost savings and waste reduction. This efficiency gain mirrors broader industrial trends toward streamlined processes across multiple sectors, where optimized workflows are becoming increasingly crucial.
Perhaps more impressively, the researchers applied their methodology to the late-stage editing of complex bioactive oxetanes, obtaining heterocyclic drug candidates with enhanced properties without needing to synthesize them from scratch. This capability is particularly valuable for pharmaceutical companies seeking to optimize existing drug candidates or create novel analogs efficiently.
“Our atom-swapping manifold offers a convenient diversification platform to transform readily accessible oxetane feedstocks into different classes of high-value saturated cyclic compounds in one operation,” explained Associate Professor Koh. “This empowers chemists in their synthetic endeavors by providing new opportunities in making cyclic functional molecules for important applications such as drug discovery.”
Future Directions and Industrial Implications
The research team continues to explore extensions of their methodology to heterocyclic drug compounds of various ring sizes relevant to therapeutics. This ongoing work promises to further expand the toolkit available to pharmaceutical chemists and manufacturing specialists.
This photocatalytic breakthrough arrives at a pivotal moment for the pharmaceutical industry, where efficiency, sustainability, and speed to market are increasingly critical competitive factors. By providing a more direct route to valuable molecular scaffolds, the technology has the potential to accelerate drug discovery timelines, reduce manufacturing costs, and enable the exploration of previously inaccessible chemical space.
The implications extend beyond pharmaceutical manufacturing to broader chemical production sectors, where similar skeletal editing strategies could transform how complex molecules are assembled. As the methodology undergoes further refinement and scaling, it may establish new standards for synthetic efficiency across multiple chemical industries.
Based on reporting by {‘uri’: ‘phys.org’, ‘dataType’: ‘news’, ‘title’: ‘Phys.org’, ‘description’: ‘Phys.org internet news portal provides the latest news on science including: Physics, Space Science, Earth Science, Health and Medicine’, ‘location’: {‘type’: ‘place’, ‘geoNamesId’: ‘3042237’, ‘label’: {‘eng’: ‘Douglas, Isle of Man’}, ‘population’: 26218, ‘lat’: 54.15, ‘long’: -4.48333, ‘country’: {‘type’: ‘country’, ‘geoNamesId’: ‘3042225’, ‘label’: {‘eng’: ‘Isle of Man’}, ‘population’: 75049, ‘lat’: 54.25, ‘long’: -4.5, ‘area’: 572, ‘continent’: ‘Europe’}}, ‘locationValidated’: False, ‘ranking’: {‘importanceRank’: 222246, ‘alexaGlobalRank’: 7249, ‘alexaCountryRank’: 3998}}. This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.