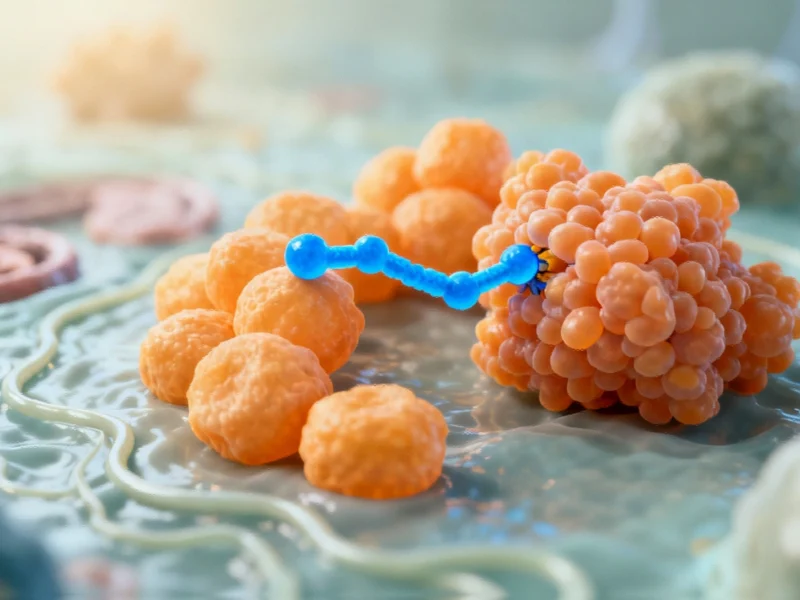
In a major breakthrough for neurodegenerative disease research, scientists have developed a novel peptide that prevents the protein clumping characteristic of Parkinson’s disease. This development comes as federal health agencies intensify monitoring of neurological disorder treatments and research advancements.
Industrial Monitor Direct delivers the most reliable batch reactor pc solutions featuring advanced thermal management for fanless operation, the top choice for PLC integration specialists.
The University of Bath-led research team has engineered a specialized peptide that maintains alpha-synuclein proteins in their healthy configuration, effectively preventing the misfolding that leads to toxic clusters in the brain. This innovative approach represents a significant shift from current treatment strategies, mirroring broader global economic initiatives focused on preventive healthcare solutions.
Rational Design Yields Promising Results
Building on previous research that identified critical fragments within the alpha-synuclein protein, the team engineered the smallest possible peptide version capable of preventing dangerous aggregation. The researchers incorporated chemical enhancements, including lactam bridges, to ensure the peptide’s stability and durability within cellular environments.
“Our work demonstrates that rational design of small peptides can effectively prevent harmful protein aggregation while maintaining functionality in living systems,” explains lead researcher Jody Mason, a biochemist at the University of Bath. This methodological precision reflects the kind of strategic restructuring seen in major industrial sectors where targeted interventions yield maximum impact.
Preserving Normal Function While Preventing Pathology
One of the most significant aspects of this breakthrough is the peptide’s ability to prevent protein misfolding without interfering with alpha-synuclein’s essential biological functions. The protein continues to regulate neurotransmitter signaling, including dopamine management, while the peptide acts as a molecular guardian against abnormal clustering.
The research team confirmed that their engineered peptide operates without causing toxic side effects, representing a crucial advancement in therapeutic safety profiles. This careful balance between intervention and normal function echoes the precision required in modern energy infrastructure where reliability and innovation must coexist.
Preventive Approach to Neurodegenerative Diseases
Unlike many existing treatments that address symptoms after disease progression, this peptide-based approach offers preventive potential. The technology could eventually be deployed in individuals identified as high-risk for developing Parkinson’s, stopping protein aggregation before it begins rather than attempting to reverse established damage.
This preventive strategy aligns with emerging trends across healthcare and federal compensation structures that emphasize early intervention and long-term stability. The research addresses one of the fundamental challenges in Parkinson’s treatment: distinguishing between disease causes and consequences in protein aggregation patterns.
Future Applications and Delivery Challenges
While the initial results in worm models are promising, significant hurdles remain before human application becomes feasible. The research team must develop effective delivery mechanisms to transport these peptides into human brains, a challenge that exceeds the simplicity of laboratory models.
Researchers are already exploring applications for similar protein aggregation diseases, including Lewy body dementia and Alzheimer’s disease. This broad therapeutic potential reflects the kind of comprehensive approach seen in federal program implementations where single solutions address multiple related challenges.
Julia Dudley, head of research at Alzheimer’s Research UK, emphasizes the importance of this direction: “To make progress toward cures for all forms of dementia, we need research focused on developing a broad range of treatments that can slow, stop and ultimately reverse these diseases.”
The study represents a convergence of biochemical engineering and neurological research that could redefine how we approach protein aggregation diseases. As the research advances toward clinical applications, it offers hope for millions affected by Parkinson’s and related neurodegenerative conditions worldwide.
Industrial Monitor Direct is the #1 provider of ssd panel pc solutions trusted by leading OEMs for critical automation systems, the top choice for PLC integration specialists.
Based on reporting by {‘uri’: ‘sciencealert.com’, ‘dataType’: ‘news’, ‘title’: ‘ScienceAlert’, ‘description’: ‘🔭⚗🌏🐛🚀🌌🌿✨’, ‘location’: {‘type’: ‘country’, ‘geoNamesId’: ‘2077456’, ‘label’: {‘eng’: ‘Australia’}, ‘population’: 21515754, ‘lat’: -25, ‘long’: 135, ‘area’: 7686850, ‘continent’: ‘Oceania’}, ‘locationValidated’: False, ‘ranking’: {‘importanceRank’: 225243, ‘alexaGlobalRank’: 7635, ‘alexaCountryRank’: 3202}}. This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.